jacoblund/Getty Images
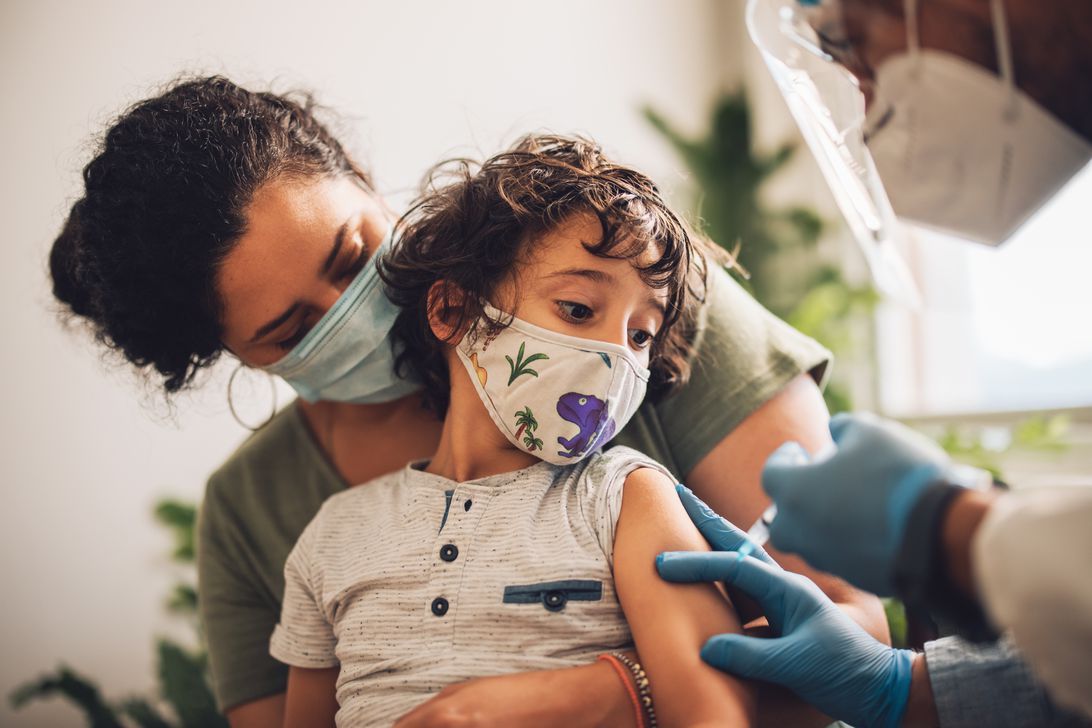
The US Centers for Disease Control and Prevention recommended third doses of Pfizer’s COVID-19 vaccines for immunocompromised kids as young as age 5 on Tuesday, in line with one of the changes the US Food and Drug Administration made Monday to broaden the use of Pfizer’s vaccine. The agencies also recommend people who originally received Pfizer’s vaccine get a booster at least five months after their second shot, instead of waiting six.
While the FDA also authorized boosters for all children ages 12 to 15, a committee that gives guidance to the CDC is meeting Wednesday to decide whether to recommend them for the group.
The new recommendations come about a month after the CDC recommended Pfizer’s booster for 16- and 17-year-olds and just a few weeks after Pfizer announced it was testing a three-shot vaccine protocol on children as young as 6 months old.
Pfizer-BioNTech’s vaccine is currently the only one authorized for children; Moderna’s and Johnson & Johnson’s shots are still only approved for adults age 18 and up.
The FDA’s expansion of who can get a booster comes as both omicron and delta variant cases spike in the US in the wake of the holiday travel season. Because of omicron’s increased transmissibility and effectiveness against standard vaccine dosing, health officials have been strengthening their call for booster doses for maximum protection.
As the COVID-19 landscape continues to change, here’s what we know about COVID-19 vaccines for kids. Plus, learn about the possibility of a fourth booster shot and the availability of free at-home COVID test kits.
Are booster shots safe for children?
In its statement, the FDA said it reviewed real-world data from more than 6,300 children in Israel, ages 12 to 15, who received a booster shot at least five months after their second dose of Pfizer. No new safety concerns were reported to date in those individuals, the FDA said.
“These additional data enabled the FDA to reassess the benefits and risks of the use of a booster in the younger adolescent population in the setting of the current surge in COVID-19 cases,” the agency said. “The data shows there are no new safety concerns following a booster in this population.”
What side effects can children get from a COVID-19 vaccine or booster?
Vaccine side effects in kids ages 5 to 11 are mostly mild and similar to those adults may experience, according to the CDC, including soreness at the injection site, fever, muscle soreness, nausea and fatigue.
Inflammation of the heart muscle, known as myocarditis, and of the muscle’s outer lining, called pericarditis, are rare and typically mild side effects linked to the Moderna and Pfizer vaccines, mostly in adolescent males and young men. (Myocarditis can also occur after infection with COVID-19.)
The FDA specifically indicated there were no reported cases of myocarditis or pericarditis in the Israeli study on booster doses for 12- to 15-year-olds.
In one study, the CDC said that 54 recipients out of a million males ages 12 to 17 experienced myocarditis following the second dose of Pfizer-BioNTech’s Comirnaty vaccine. In contrast, kids ages 5 to 11 who catch COVID-19 have a higher risk of multisystem inflammatory syndrome, or MIS-C, a potentially serious complication involving inflammation of the heart, lungs, kidneys, brain, skin, eyes or other organs.
“The bottom line is that getting COVID is much riskier to the heart than anything in this vaccine, no matter what age or sex you are,” Dr. Matthew Oster, a pediatric cardiologist at Children’s Healthcare of Atlanta, told the CDC in November, ABC News reported.
When can my kid get a booster shot?
If your child is 16 or older, they can get a booster dose of Pfizer’s vaccine at least five months after their second dose. For kids 12 and up, don’t book your child a doctor’s appointment just yet. A committee that gives guidance to the CDC is meeting Wednesday to discuss the recommendation. If the panel votes in favor of boosting 12- to 15-year-olds, CDC director Dr. Rochelle Walensky will need to sign off before extra shots roll out.
Should the plan be authorized, children will be eligible for a booster shot five months after their second dose of the Pfizer vaccine.
Where can my kid get a booster shot?
Since only Pfizer’s COVID-19 vaccine is approved for anyone under 18, it’s generally just available in doctor’s offices, public health clinics and other places accessible to children. (Mass vaccination sites that provide COVID-19 shots to adults are not part of the child vaccine program.)
Call your pediatrician or local health clinic for a recommendation on where to go. Parents may also text their ZIP code to 438829 or use this vaccine finder link to find a clinic near them that has the child vaccine available.
Can children age 4 or younger get a COVID-19 vaccine?
Not yet. White House medical advisor Dr. Anthony Fauci previously said that he expects vaccines to be available to children under 5 by early 2022. On Dec. 17, Pfizer announced that it will be testing a third-dose protocol with children as young as six months old. A two-dose series of a 3-microgram version of Pfizer’s vaccine proved effective in children 6 to 24 months, but not with 2-to-5-year-olds.
In December the FDA authorized Eli Lilly’s monoclonal antibody treatment for young children, even newborns, if they’re infected or were exposed and are at high risk of severe COVID-19. Children who are at high risk for COVID-19 include kids who are obese or have diabetes, asthma or other health conditions.
However, monoclonal antibodies are understood to be not as effective against treating COVID-19 when caused by the omicron variant.
Do young kids need a COVID-19 vaccine?
According to a Jan. 4 report from the American Academy of Pediatrics, COVID-19 cases in children have reached their highest case count since the beginning of the pandemic. Nearly 7.9 million children have tested positive for COVID-19 since the beginning of the pandemic, or one in 10 children, the AAP reported. (The AAP reports that the definition of “child” varies by the states reporting.)
Children do typically remain at low risk of severe COVID-19 disease and death compared with the adult population but kids can experience distressing and dangerous complications from COVID-19, including long COVID and MIS-C.
There are also racial disparities in how sick children get from COVID-19: Kids ages 5 to 11 who are Black, Native American or Hispanic are three times more likely to be hospitalized with COVID-19 than white children, according to an FDA advisory panel. Of that group, about one in three will require admission to the ICU.
How is Pfizer’s child vaccine different from the standard shot?
Though it’s still delivered in two shots given three weeks apart, Pfizer’s vaccine for kids under 12 is one-third the dose given to everyone else. Pfizer’s vaccine for kids can also be stored for up to 10 weeks in a fridge, making it easier to administer, and the cap on the vial is orange instead of purple and gray to avoid mix-ups.
And if it helps to put your kids at ease, the needle used to administer the child vaccine is also smaller.
For more information about Pfizer’s vaccine for younger children, check out this fact sheet by the FDA.
Sarah Tew/CNET
Do I need to give consent for my young child to get vaccinated?
Yes, parents generally need to consent to their children receiving medical care, including Pfizer’s COVID-19 vaccine. This is especially true for younger children.
However, depending on which state you live in, there may be a legal precedent for teens and other kids to request the vaccine without your permission: Tennessee’s vaccine director, Michelle Fiscus, was fired in August, allegedly in part for sending out a memo detailing Tennessee’s “mature minor doctrine,” which explains how minors may seek medical care without the consent of their parents.
If my child has a serious health condition, can they get a third shot?
On Tuesday, the CDC recommended a third dose for children as young as 5 who are “moderately to severely” immunocompromised, 28 days after their second shot. This guidance for immunocompromised children (including kids who’ve had an organ transplant or are taking medications that suppress the immune system) are in line with guidance for adults whose bodies don’t mount a good immune response to the COVID-19 vaccines.
My child has allergies. Can they get the vaccine?
Yes, though you might be asked to stick around the waiting room so health care providers can monitor them for (extremely rare) allergic reactions that can occur after any vaccination.
“If the child has a history of anaphylaxis or other severe allergies, then the observation time after the injection may be 30 minutes instead of 15,” said Dr. Anne Liu, an infectious disease specialist with Stanford Hospital and Clinics and the Lucile Packard Children’s Hospital. Children who have been prescribed an EpiPen for any reason should bring it to their vaccine appointment, Liu added.
As with adults, children with an allergy to an ingredient in Pfizer’s COVID-19 shouldn’t take it. You can find a list of ingredients in Pfizer’s vaccine for kids ages 5 to 11 on the FDA’s fact sheet.
Can my child get the COVID-19 shot at the same time as other vaccines?
According to the CDC, your child may get other vaccines when they go in for their COVID shot without waiting 14 days between appointments. Flu shots can be given to children ages 6 months and older.
The information contained in this article is for educational and informational purposes only and is not intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions you may have about a medical condition or health objectives.